
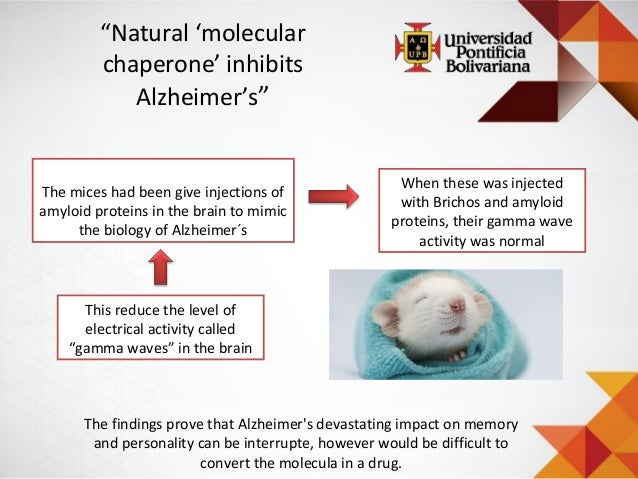
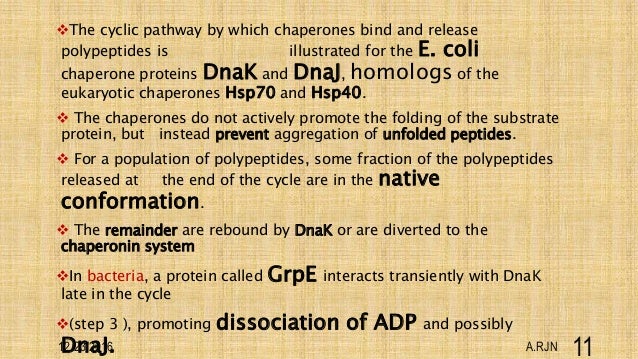
It was quickly determined that this "chaperone" protein directing correct assembly was identical to one of the many proteins expressed at high levels when cells are grown at high temperatures (hence the common alternative name, "heat-shock protein," or Hsp). A new protein was identified that was required for correct folding of a large enzyme complex in chloroplasts, yet the mysterious protein was not associated with the final assembled complex. Discovery of ChaperonesĬhaperones were originally identified in the mid-1980s from studies of protein folding and assembly in plant chloroplasts. Chaperones are found in all types of cells and cellular compartments, and have a wide range of binding specificities and functional roles. The misfolded or unfolded polypeptide chains to which chaperones bind are said to be "non-native," meaning that they are not folded into their functional conformation. All proteins are created at the ribosome as straight chains of amino acids, but must be folded into a precise, three-dimensional shape (conformation) in order to perform their specific functions. Molecular chaperones are proteins and protein complexes that bind to misfolded or unfolded polypeptide chains and affect the subsequent folding processes of these chains.


 0 kommentar(er)
0 kommentar(er)
